PROMPPT is a 5-year research programme funded from March 2019 by the UK National Institute for Health Research (NIHR), to develop and test a pharmacist-led primary care intervention.
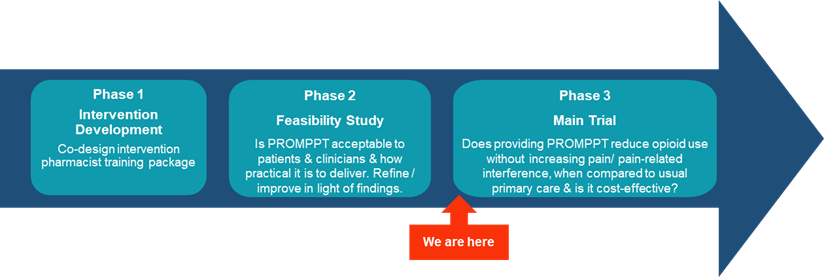
The research programme is now in phase 3, a large multicentre cluster randomised controlled trial (cluster RCT) that aims to test the clinical and cost-effectiveness of providing the PROMPPT pain review and training package. This trial will investigate whether, compared with usual care, providing a PROMPPT pain review for patients prescribed long-term opioids for persistent pain reduces opioid use (where a reduction is at least 25%, on average), without making pain/pain-related interference worse.
The PROMPPT Trial
The PROMPPT trial aims to test the effectiveness of the PROMPPT intervention (the training package, the pain review, patient facing information and documents) to reduce opioid use in patients prescribed long-term opioids for persistent pain.
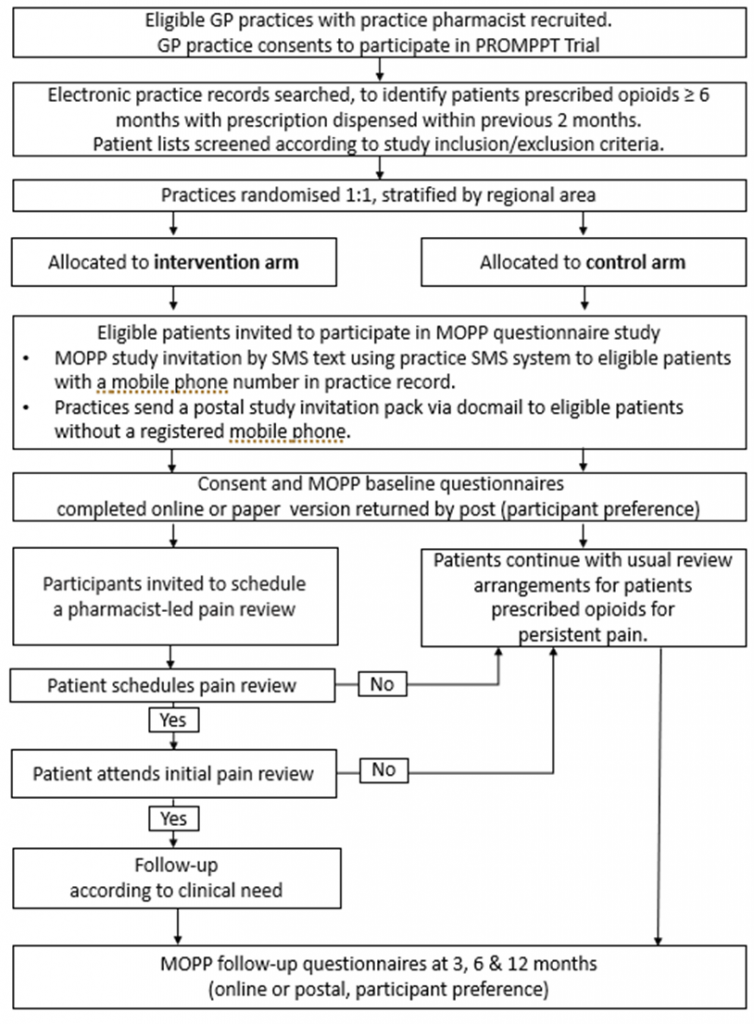
The PROMPPT trial is made up of 3 separate components (a questionnaire study, the pain review and the process evaluation) that will help to test the intervention:

How many patients will take part in the trial?
The trial aims to recruit 896 participants from approximately 30-34 GP practices located across three areas; West Midlands, East Midlands and Wessex. Each intervention GP practice (approximately 15-17), will recruit and deliver pain reviews to approximately 30 participants (this number will depend on how may participants consent to take part in the MOPP questionnaire study from the practice). As a clinical champion, it is anticipated that you will help to support around 6 intervention practices in your area across the trial.
How will GP practices be identified?
The PROMPPT trial will be advertised to the GP practices across the three areas with the support of the local Clinical Research Network’s (CRN).
What happens once a GP practice agrees to take part in the trial?
Once a practice initially expresses an interest in taking part in the PROMPPT trial and finally agrees to be involved, the lead GP, pharmacist delivering the pain reviews and the practice manager will attend a site initiation visit (SIV) with the PROMPPT research team.
During the SIV, the research team will:
- Introduce the PROMPPT trial
- Explain the trial processes and trial flowchart (See below)
- Explain the participant identification process
- Outline the PROMPPT trial timeline (See below)
- Ask the practice to complete the baseline practice profile form
- Answer any questions that the practice may have
What happens when the practices are randomised?
Before GP practices are randomised to intervention or control arms, they will attend the SIV with the research team, and complete the baseline practice profile form. Randomisation of practices to intervention or control arms coincides with the e-learning being released to the practice pharmacists (intervention arm only).
Practices that are randomised to the control arm will invite patients to take part in the MOPP questionnaire study and continue to provide usual review arrangements for their patients prescribed opioids for persistent pain.
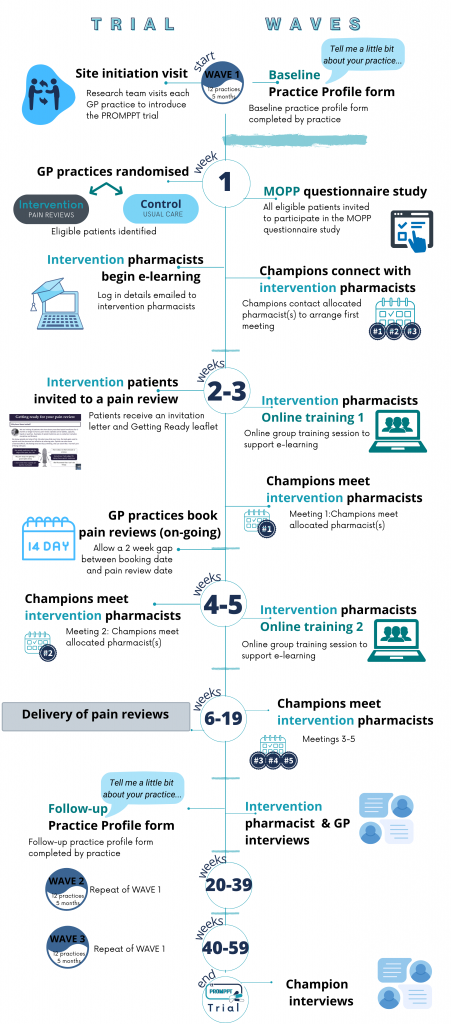
Will all GP practices and patients be recruited at the same time?
To help with recruitment and delivery of the trial, there will be three waves of recruitment. The first wave will aim to recruit approximately 12 practices, with the second wave aiming to recruit a further 12 practices 5 months later. The third wave of recruitment will again be 5 months after the second but the number of practices will depend on the recruitment figures.
Once a pharmacist has completed the e-learning and the 2 group sessions, they will be encouraged to deliver pain reviews as soon as possible. The pharmacist will have 3 months to deliver their allocated pain reviews (first assessment) and follow-ups. If follow-ups are required following the end of the 3-month period, these can take place but are no longer considered as part of the research and the pharmacist is not required to send a case report form (CRF) to the research team.